Surface active agents, also known as surfactants, are compounds that can significantly reduce the surface tension (surface tension) or interfacial tension (interfacial tension) between two liquids, liquid-gas, or liquid-solid interfaces. In daily life, various detergents such as soaps and cleansers contain surfactants as active ingredients. So what is the principle behind their action?
Structure of personal care surfactants
Surfactants generally consist of two parts: hydrophilic groups, which consist of one or more polar groups (hydrophilic groups); and lipophilic groups, which consist of non-polar hydrocarbon chains (lipophilic groups).
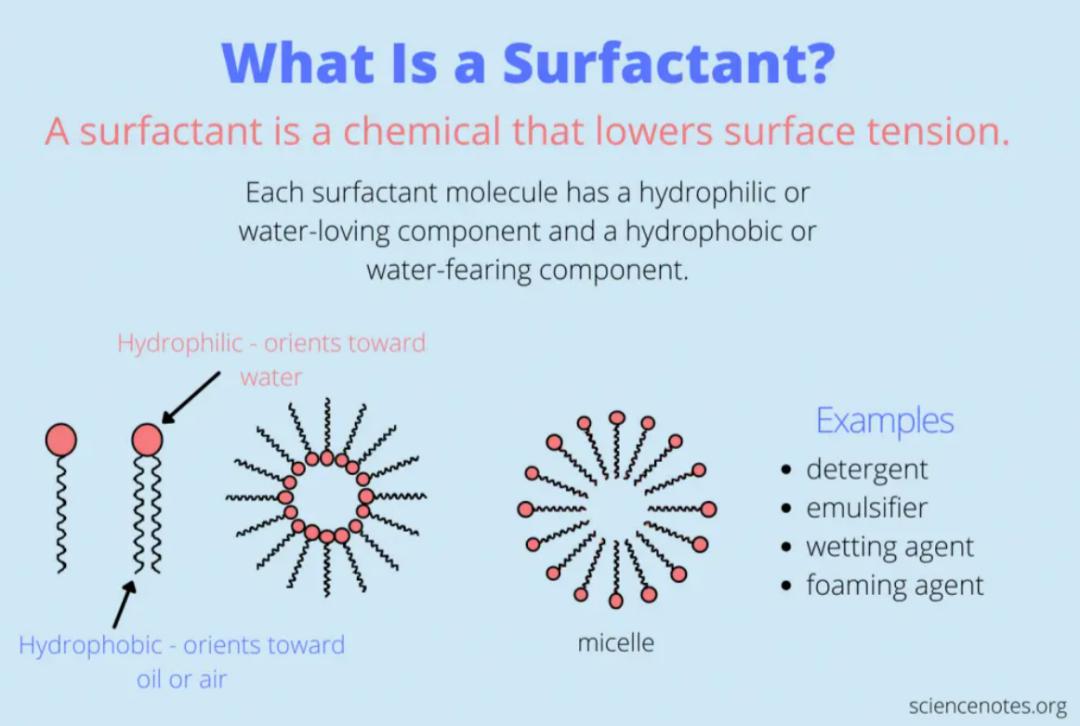
Schematic diagram of surfactant structure (image source:sciencenotes.org).
Types of personal care surfactants
According to the molecular structure and dissociation properties of polar groups, surfactants can be divided into anionic surfactants, cationic surfactants, amphoteric surfactants, and nonionic surfactants.
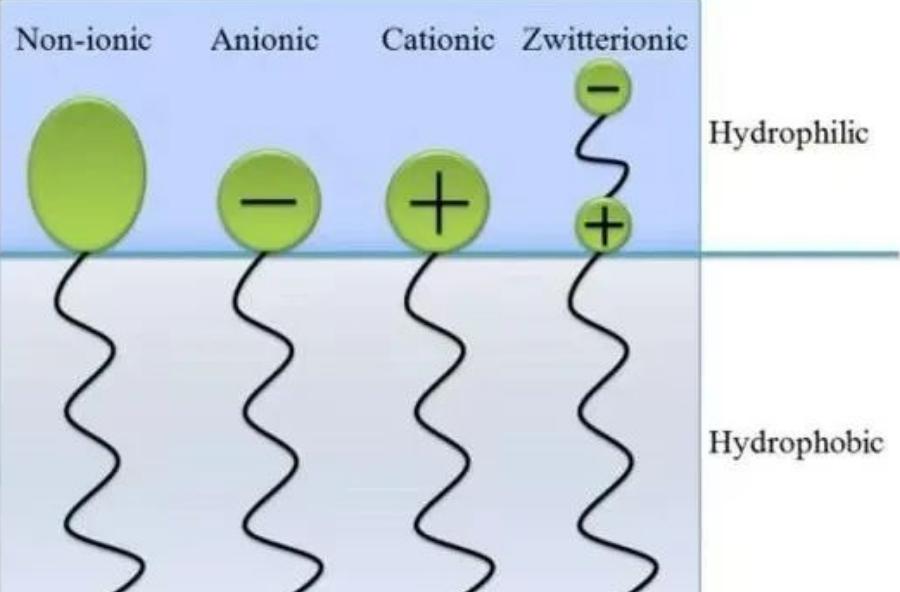
Four types of surfactant structures
Surfactants with charged hydrophilic ends are known as ionic surfactants, classified into three categories based on the nature of their charges:
Cationic surface active agent
Cationic surfactants are mainly divided into ammonium salt types and quaternary ammonium salt types. They are primarily utilized for their antimicrobial and disinfectant properties.
Anionic Surfactants
Anionic surfactant typically contain sulfate, sulfonate, or carboxylate groups. They find applications in various personal care products due to their cleansing properties.
Zwitterionic/Amphoteric/Ampholytic Surfactants
This category of surfactants has both cationic and anionic groups on their hydrophilic ends, resulting in a net charge of zero. They can react both with acids and bases. In acidic solutions, they behave as cations, while in alkaline solutions, they behave as anions. There are four main types: betaines, amino acids, imidazolines, and amine oxides.
Non-Ionic Surfactants (Nonionics)
Non-ionic surfactants have a hydrophilic end without a charge. Their hydrophilic-lipophilic balance (HLB) measures their affinity for water or oil. Higher HLB values indicate greater oil affinity, while lower values indicate water affinity. Non-ionic surfactants are generally considered mild and do not cause protein denaturation.
Principles of Action of Personal Care Surfactants
Surfactants serve various functions in personal care products by reducing liquid surface tension, which tends to minimize the surface area due to inward molecular movement. For instance, oil and water do not naturally mix, but the presence of surfactants reduces the surface tension on both oil and water interfaces, facilitating their blending. The molecular structure of surfactants allows them to adsorb oil with hydrophobic groups and dissolve in water with hydrophilic groups, effectively combining oil and water for cleansing purposes.
The extensive use of surfactants in personal care products, including facial cleansers, body washes, shampoos, toothpaste, etc., is attributed to their emulsifying, cleansing, foaming, solubilizing, antibacterial, antistatic, and dispersing properties.
Personal care surfactants products play a crucial role by reducing water surface tension, enabling wetting, emulsification, dispersion, foaming or defoaming, solubilization, and other essential actions. However, individuals with different skin types need to choose surfactants based on their personal conditions.